![]()
How many steroisomeric tetrabromides will form?
How many steroisomeric tetrabromides will form?
How many steroisomeric pentabromides will form?
A. I, followed by II
B. II, followed by I
C. III
A. I, followed by II
B. II, followed by III
C. III, followed by II
D. IV
1108. Which compound is a possible product from addition of Br2 to 1-butene?
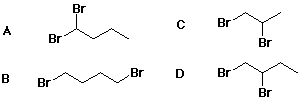
A, B, C, D
1109. According to Markovnikov's rule, addition of water to 1-butene should give a
A. primary alcohol
B. secondary alcohol
C. tertiary alcohol
D. none of the above
1110. If a hypothetical reagent XY gave a syn addition to cyclopentene, the product would be
A. cis
B. trans
C. a mixture of cis and trans
D. neither cis nor trans
1111. Addition of Br2 to 1-butene would give 1,2-dibromobutane which is
A. achiral
B. racemic
C. meso
D. optically active
1112. Addition of Br2 to cyclopentene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
1113. Addition of Br2 to cis-2-butene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
1114. Addition of Br2 to trans-2-butene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
1115. Addition of OsO4 to cyclopentene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
1116. Addition of BH3 followed by H2O2 to trans-2-butene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
1117. Addition of BH3 followed by H2O2 to 1,2-dimethylcyclopentene would give a product which is
A. achiral
B. racemic
C. meso
D. optically active
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..