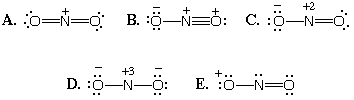
1027. Which Lewis dot structure for NO2+ has the lowest energy?
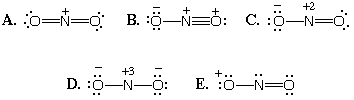
A.
B.
C.
D.
E.
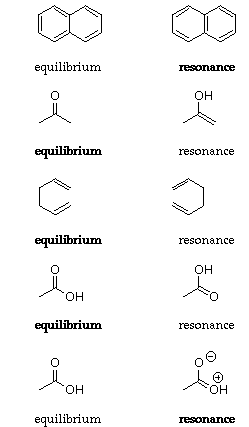
Which resonance structure has the lowest energy?
A. 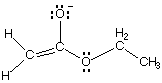
B. 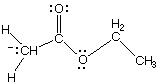
A. The double bond would have the same strength as the single bond.
B. The double bond would have less than twice the strength than the corresponding single bond.
C. The double bond would have exactly twice the strength of the single bond.
D. The double bond would have more than twice the strength of the single bond.
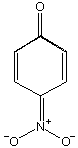
Is p-nitrophenol more or less acidic than phenol? more
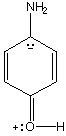
Is p-aminophenol a weaker or stronger base than aniline? stronger
1089. The structure below that has an error is:
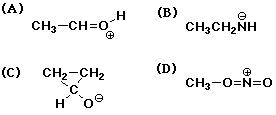
A B C D
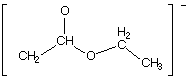
1090. Converting between the two resonance forms below (left to right) involves electron pushing of:
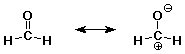
(A) a lone pair from O to C
(B) a lone pair from C to O
(C) a pi bond to C
(D) a pi bond to O
1121. Chemists determine the activation energy for a reaction by
A. measuring product amounts
B. measuring rates
C. calculating from bond dissociation energies
D. calculating from
H values
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..