Inorganic Chemistry: Electronic Structure
3035) Using the Tanabe-Sugano diagram below for the d2 electronic
configuration, how many spin-allowed transitions would be expected in the
electronic absorption spectrum?
0, 1, 2, 3

3036) Demonstration: A 1M Mn2+ solution [Mn(H2O)6]2+
in a large pathlength container is observed to be very pale pink in color
(almost colorless).
From the Tanabe - Sugano diagram below, the electronic transitions for this
Mn complex lie on which side of the vertical line?
left, right

3057) All octahedral (Oh) and tetrahedral (Td) complexes
with d1 and d9 electronic configurations have 2T2(g) or 2E(g)
ground states. This typifies the relationship that the Oh dn
energy level diagram is the same as that for:
Td dn, Td d10-n
3037) After showing that the ground state term symbols of d1
and d2 free ions are 2D and 3F, respectively, which of the following
statements is true for other transition metal free ions?
dn and d10-n have the same ground state term symbol,
dn and d10-n do not have the same ground state term symbol
3038) There are how many d electrons in the [Mn(H2O)6]2+
complex?
5, 6, 7
3039) From crystal field theory, which set of d orbitals
is higher in energy in an Oh complex?
eg, t2g
In a Td complex?
e, t2
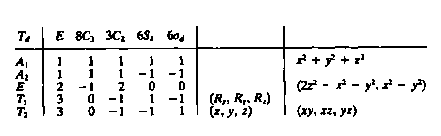
3040) Using the character table below, to what irreducible representation(s)
do the d-orbitals belong in a tetrahedral complex?
A1, A2, E, T1, T2

3041) In the Td molecule MX4, the A1 vibrational mode corresponds to all
the M-X bonds stretching the same distance simultaneously. With which spectroscopic
technique is this vibrational mode observable?
IR, Raman, neither, both
Which of the following salts (high spin for paramagnetic samples) will have
the strongest attraction to a magnet?
MnSO4, CoSO4, ZnSO4
Demonstration: Capsules of each of these materials are suspended
from a thread. Bringing a strong magnet close to each capsule will produce
the largest response from the Mn sample (5 unpaired electrons), next largest
response from the Co sample (3 unpaired electrons), and negligible response
from the Zn sample (diamagnetic).
3042) Demonstration:
[Ni(H2O)6]2+ (green) + NH3 --->
blue complex
[Ni(H2O)6]2+ (green) + en ---> violet
complex
From this kind of "eyeball spectroscopy," which ligand gives rise
to the largest D0 value (d-orbital splitting)?
H2O, NH3, en
3043) If V(CO)6 is an 18-electron complex,
what is the charge on the complex, if any?
+1, 0, -1
3044) In constructing an MO diagram for CO, for which
element are the 2p atomic orbitals at higher energy?
C, O
3045) In a metal carbonyl complex, as more electron density
moves from the metal d-orbitals to the CO pi* orbital (d --> pi*), what
happens to the CO stretching frequency?
it increases, it decreases, it stays the same
3046) In the neutral complex Fe(h5-C5H5)2,
is the 18-electron rule obeyed?
Yes, No
3047) For the unbalanced reaction:
ReO4- + HCl --H2--> (reduction) [Re2Cl8]2-
What is the oxidation state of the Re atoms in the product?
II, III, IV
3048) Photo-excitation promotes an electron from the HOMO
(highest occupied molecular orbital) to the LUMO (lowest unoccupied molecular
orbital) of a molecule (see diagram below). Which is the stronger oxidant?
ground state, excited state
Which is the stronger reductant?
ground state, excited state

3058) Although pure diamonds are colorless, some diamonds have color because
of dopants. The Hope diamond, for example, is blue due to nitrogen (N) dopants.
Based on its position in the periodic table, N in diamond acts as a(n):
donor, acceptor
Complete the equilibrium reaction shown below for this dopant:

The charge on the product ion is:
positive, negative
The ionization of N leads to the production of a(n):
electron, hole
Which arrow shown below represents the electronic transition corresponding
to the ionization of N?

A, B
According to LeChatlier's Principle, adding boron (an acceptor) to N-doped
diamond will shift the equilibrium of equation 1 toward the:
products, reactants
(A similar set of questions could be asked about doping with B, which can
produce yellow diamonds.)



More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..






![]()

![]()
![]()
![]()