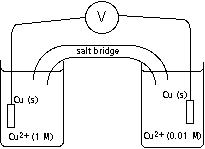
the solutions retain their respective colors, the dark solution becomes darker and the light solution becomes lighter, the solutions become indistinguishable in color
The entropy of the system will
increase, decrease, stay the same
The enthalpy of the system will
increase, decrease, stay the same
Cu2+ (in 1 M soln.) ---> Cu(s) and Cu(s) --->Cu2+ (in 0.01 M soln.),
Cu2+ (in 0.01 M soln.) ---> Cu(s) and Cu(s) --->Cu2+ (in 1 M soln.)
Will a voltage be measured?
yes, no
If the pictured solutions are mixed and then divided into separate cells, will a voltage be measured?
yes, no
When unmixed, in which direction do the electrons travel when current is allowed to flow?
right, left
To which cell will the positively charged ions in the salt bridge flow?
right, left
Demonstration: With a concentration cell, demonstrate the answers
to each of the questions above.

Ag+ + e- --->Ag E°= 0.80 V;
Cu2+ + 2e- ---> Cu E°= 0.34 V
Will Ag react with Cu2+?
yes, no
Will Cu react with Ag+?
yes, no
Demonstration: Cu + 2 Ag+ ---> Cu2+ + 2 Ag Place
a sheet of copper into a AgNO3 solution. The submerged copper
will be plated with silver at the end of the reaction.
Demonstration: Construct a galvanic cell: ![]()
Measure the voltage.
If water is added to the Cu2+ cell, how will the voltage be affected?
voltage will increase, voltage will decrease, no change
If ammonia is added to the Cu2+ cell to form an amine complex, how will the voltage be affected?
voltage will increase, voltage will decrease, no change
If Cl- solution is added to the Ag+ half cell to precipitate AgCl, how will the voltage be affected?
voltage will increase, voltage will decrease, no change
As current passes, the voltage
increases, decreases, stays constant
at low temperature for a short time, at high temperature for a long time
Which way do electrons want to go in a p-n junction to establish equilibrium?
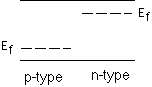
n-type side to p-type side, p-type side to n-type side
Which p-n junction will give the larger voltage?
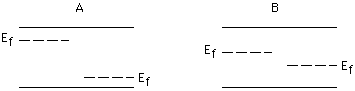
A, B
Consider a p-n junction formed by ZnSe (band gap 2.7 eV) and GaAs (band
gap 1.4 eV). Which side of the junction is ZnSe?
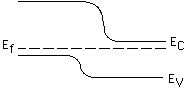
left, right
Which side of the junction is p-type?
left, right
![]()
Which of the following reactions could be used as the anode (oxidation)?
![]()
A, B
zero volts
0.03 volts
0.06 volts
0.12 volts
238. Both leads of an LED conductivity probe are dipped in water, dried, and then dipped in mercury. In which liquid(s) will the LED light up?
H2O, Hg, both, neither
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..