Bonding and Structure
7. (Unit cells; Ch. 3 "Companion") A corner
atom is part of how many unit cells?
.

1, 2, 4, 8
At-Seat Demonstration: cubes like dice can be handed out for students
to examine for this question.
8. (Coordination number; Ch. 5 "Companion")
In the two-dimensional square packing shown, what is the coordination number
of the central atom?

2, 4, 6
9. (Coordination number; Ch. 5 "Companion")
At-Seat Demonstration: Give students 10 pennies (students can share
a set of ten) and ask them to pack them at their desks to determine the
largest coordination number in two dimensions.
4, 6, 8, 9
25. (Lewis dot structures, octet rule) What is a correct
Lewis dot representation of carbon monoxide?

26. (Lewis dot structures,
bonding) Compare O-O and O=O. Is O=O expected to be
stronger, weaker, or the same strength?
Is O=O expected to be
longer, shorter, or the same length?
27. (VSEPR) Demonstration
5.3 "Companion": Provide students with small plastic tetrahedral
and octahedral models to share during VSEPR lectures. With four electron
pairs, can the pairs be equally spaced and farther apart than the 90°
of a square arrangement?
yes, no
Determine the Lewis dot structure of NH3 and use the models
to determine its molecular geometry.
linear, bent, pyramidal
Repeat for H2O.
linear, bent, pyramidal
A tetrahedron can be inscribed inside a cube, represented in the layer
sequence formalism shown below. If sphere 1 is part of the tetrahedron,
which other three spheres are as well?

2, 3, 4
3, 5, 7
3, 6, 8
An octahedron can be inscribed inside a cube. If spheres 1 and 6 (below)
are part of the octahedron, where do the other four spheres go?

z = 1/4, 1/2, 3/4
What is their arrangement?

A, B, C
28. (VSEPR) At-Seat Demonstration
(use models described above): The center of a tetrahedron resting on three
bonds
is midway from top to bottom, is closer to the bottom, is closer
to the top
The center of a tetrahedron balanced on two bonds
is midway from top to bottom, is closer to the bottom, is closer
to the top
The center of an octahedron resting on three bonds
is midway from top to bottom, is closer to the bottom, is closer
to the top
The center of an octahedron balanced on one bond
is midway from top to bottom, is closer to the bottom, is closer
to the top
This can be connected to location of centers of octahedral and tetrahedral
holes in close-packing arrangements of atoms/ions.
29. (Close packed spheres,
holes; Ch. 5 "Companion") The figure below is a ZnS (zinc blende)
layer sequence. The atom indicated by an arrow sits in one of the tetrahedral
holes of the structure. Which atoms form this hole?

1,4,7,8
1,3,4,7
1,3,6,7
30. (Close packed spheres,
holes; Ch. 5 "Companion") In the zinc blende layer sequence below,
what fraction of the tetrahedral holes are filled?

1/8, 1/4, 1/2, all
31. (Close packed spheres,
holes; Ch. 5 "Companion") C60 (buckyball) is cubic
closest packed (face-centered cubic) in its crystalline form. If you insert
potassium atoms into all the tetrahedral and octahedral holes of the C60
structure, the formula would become KxC60 . What is
the value of x?
1, 2, 3, other
32. (Polarity; dipole moments)
Demonstration: Beakers of water, carbon tetrachloride, and dry ice
(carbon dioxide) are to be placed into a microwave oven. Based on dipole
moment, which are predicted to be heated by the microwave radiation (measure
temperature with digital thermometer before and after)?
water, carbon tetrachloride, carbon dioxide
33. (Bands; Ch. 7 "Companion")
The 3s band of solid Na will have as many orbitals (each delocalized over
the entire solid) as the number of 3s orbitals from Na atoms in the sample.
If each orbital of the band can hold two, spin-paired 3s valence electrons,
to what extent will the band be filled?

completely empty, half filled, completely filled
To what extent will the Na 2s band be filled?
completely empty, half filled, completely filled
Which is the best electronic band population for good electrical conductivity
(metallic behavior), if a net flow of electrons through a band is needed?
Demonstration 7.1 "Companion": A requirement for electrical conductivity
is net electron flow in a particular direction in a sample. At what capacity
should the band be filled with electrons to best promote electrical conductivity?
Have three jars sealed tightly: one empty (represents empty band), one partially-filled
(represents partially filled band) and one completely filled (represents
filled band). If the filler (sand or packing peanuts, e.g.) represents electrons
and tipping the jar represents the application of a voltage, which jar(s)
will exhibit net motion of the filler?
empty, partially filled, filled
In which part of the filled portion of the band is net motion greatest?
top, middle, bottom
35. (Bands; Ch. 7 "Companion")
Which of these band diagrams is consistent with diamond being an electrical
insulator?

A, B, C
Another approach is to have students determine which bands are filled to
which extent, and then decide if each band scenario in turn (A, B, then
C) corresponds correctly to diamond's being an electrical insulator rather
than a conductor (metal). The s, p combination band can be related to sp3
hybrid orbitals if desired.
36. (Semiconductors; Ch. 7
"Companion") When an electron falls back into a bond (localized
picture) or valence band (delocalized picture), what change in energy occurs?
energy is released, energy is absorbed, no change in energy occurs
38. (Band gap energy, spectroscopy,
semiconductors; Ch. 7 "Companion") Setup: Band gap energy has
been introduced in a localized picture: it can be defined as the energy
needed to remove an electron from a bond in the solid, enabling the electron
to move freely through the solid to conduct electricity. When itinerant
electrons return to such a one-electron bond, the band gap energy can be
released as a photon. The band gap energy is to a first approximation expected
to increase as the bonds become stronger and shorter and the electrons are
held more tightly. The group 14 elements illustrate this effect with diamond
being an electrical insulator, silicon and germanium (longer, weaker bonds
in the same diamond structure) being semiconductors, and tin being a metal.
Demonstration 7.11 "Companion": A trio of related predictions:
what will happen to interatomic spacing, band gap energy, and the color
of the light emitted when an orange LED is cooled in liquid nitrogen. On
cooling,
atoms will get closer together, atoms will get farther apart
band gap energy increases, band gap energy decreases
color of light will become more red, color of light will become more
yellow
42. (Elemental analysis, diffraction,
solid solutions; Ch. 3 & 4 "Companion") Sample A is an equimolar
physical mixture of Si and Ge. Sample B is a Si0.5Ge0.5
solid solution. Which measurements will be identical and which different
for the two samples?
elemental analysis: same, different
x-ray diffraction: same, different
absorption spectrum: same, different
50. (Layer sequences; Ch. 3 & 5 "Companion")
How many atoms are in the following layer sequence of the diamond structure?

4, 6, 8, 13
What is the coordination number?
4, 6, 8
What is the coordination geometry?
tetrahedral, square, octahedral, cubic
54. (Diffraction, optical
transforms; Ch. 4 "Companion," Demonstration 4.1) The equation
dx = lL defines the relationship between incident wavelength l, spacing
of repeating features d, spacing between diffracted spots x, and distance
between transform slide and screen L. Consider which variables are directly
and inversely related to predict the following.
When the wavelength is decreased from red to green (demonstration with 633
and 543 nm HeNe lasers), should the spacing between diffraction spots
increase, decrease, stay the same
(This relationship can also be seen by having students view a point source
of white light through their personal transform slide, as described in Demonstration
4.1, and having them note which part of the full spectrum they observe for
each diffracted spot, violet or red, is giving rise to the largest spacing).
When the distance between slide and screen is increased, the spacing between
diffraction spots should
increase, decrease, stay the same
When the spacing between features on the transform slide increases, the
spacing between diffraction spots should
increase, decrease, stay the same

65. (Unit cells, layer sequences, close packed spheres;
Ch. 3 & 5 "Companion") A layer sequence for an FCC = CCP metal
is shown below.

A face diagonal passes through the center of atom 4 and the center(s)
of which other atom(s)?
1, 2, 5, 11 Also correct: 8, 12 & 9, 10
A body diagonal passes through the center of atom 4 and the center(s)
of which other atom(s)?
2, 5, 11, 14
A close-packed plane is comprised of six atoms. If atoms 2, 4, 5 are
three of the six atoms, which other three atoms are need to define the plane?
11, 13, 14; 6, 9, 13; 7, 8, 12; 6, 9, 10
71. (Organic chemistry) In
the shorthand for organic molecules, octane is written as follows:

How many hydrogen atoms are present in this molecule?
16, 18, 20
How many hydrogen atoms are present in ethene,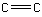 ?
?
2, 4, 6, 8
73. (Chirality) Do enantiomers
have the same density?
yes, no
melting point?
yes, no
If acids, the same pKa?
yes, no
74. (Chirality) How many stereogenic
carbon atoms are present in the glucose molecule pictured below?

3, 4, 5, 6
76. (Polymers) Some polymerization
reaction conditions lead largely to linear chains of polyethylene, A, and
other conditions cause considerable branching, B. Which structure corresponds
to high density polyethylene (HDPE)?

A, B
86. (Semiconductors, bands; Ch.8 "Companion")
Pictured below is an energy band diagram for silicon.

When doped into Si, Al is a(n)
donor, acceptor
When doped into Si, P is a(n)
donor, acceptor
Which energy level corresponds to Al?
A, B
Which energy level corresponds to P?
A, B
87. (Semiconductors, doping; Ch 8 "Companion")
Which is a weaker acceptor (analogous to weaker acid)
In, Cu
Which is a weaker donor (analogous to weaker base)?
As, Mn

92. (Close packed spheres,
holes) Demonstration: If four identical spheres are placed in contact to
form a tetrahedral hole, can an identical sphere fit into the hole?

yes, no
93. (Close packed spheres,
holes) Demonstration: Which is a smaller hole?
One formed by a tetrahedron of identical spheres, one formed
by an octahedron of identical spheres
A sphere that fits snugly into an octahedron can be shown not to fit
into a tetrahedral hole.
94. (Bonding) Demonstration:
Obtain samples of copper and silicon. Hit each with a hammer. Which would
be predicted to have directional covalent bonds?
Cu, Si
97. (Chelation) What is the
maximum number of ethylenediamine bidentate ligands that can bond to Ni
to form an octahedral complex?
2, 3, 6
104. (Organic chemistry, bonding,
Lewis dot structure) To complete the Lewis dot structure of benzene, how
many double bonds must be added?

1, 2, 3, 6
112. (Density, close packed
spheres) Demonstration: Which is denser?
Stacking a second close-packed layer of spheres directly atop a close-packed
layer below, Stacking a second close-packed layer of spheres in the
depressions formed by spheres in the close-packed layer below.
114. (Plastic deformation,
slip planes; Ch. 6 "Companion") Which situation allows easiest
slippage of planes of atoms past one another, as sketched below?

identical spheres in both layers, a layer with a large impurity
atom, a layer with a small impurity atom
Which of the two impurities acts like a "speed bump" and which
like a "pothole," in impeding movement along slip planes? (see
figure above)
115. (Electronegativity, bonding, band gap energy; Ch.
7 "Companion") The atoms below have the following electronegativities:

The three isoelectronic semiconductors Ge, GaAs, and ZnSe all have roughly
the same size unit cell and internuclear separation (exclusively Ge-Ge,
Ga-As, and Zn-Se bonds, respectively). If under these conditions, band gap
energy increases with ionic character, which isoelectronic solid should
have the largest band gap energy?
Ge, GaAs, ZnSe
117. (Chelation, coordination
chemistry, chirality) At-Seat Demonstration: Pass out three small
rubber bands with the plastic octahedra (see problem 27). Instruct students
to share two octahedra and six rubber bands. Have them make mirror image
tris chelate complexes, using the rubber band as a chelating ligand. Are
these superimposable?
yes, no
118. (Tetrahedron, chirality)
At-Seat Demonstration: Four colors of circular paper stickers (green,
yellow, pink, and orange, e.g.) can be obtained on long sheets. Instruct
students to share two tetrahedra and two stickers of each of the four colors.
Have them make mirror image tetrahedra with a different color on each leg
(representing four different substituents). Are these superimposable?
yes, no
Repeat using two different colors (two substituents identical). Are these
superimposable?
yes, no




More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..


![]()









![]()
![]() ?
?






![]()
![]()
![]()