Atoms, Molecules, Extended Structures and Stoichiometry
1. (Isotopes) Consider the Br2 molecule. If
there are two common isotopes, 79Br and 81Br, how
many physically distinguishable combinations of Br atoms are there in Br2?

2, 3, 4
2. (Isotopes, natural abundance, probabilities, mass spectrometry)
If the two Br isotopes, 79Br and 81Br, each has a
natural abundance of about 50%, what is the most likely molecular weight
of Br2 molecules?
158 amu, 160 amu, 162 amu, all three are equally likely
Demonstration: Students in class flip coins twice in succession and
the numbers of two heads, two tails, and one head/one tail flips can be
tabulated by show of hands to make a connection with probabilities of independent
events being multiplicative.
Which is the expected mass spectrum of the parent cation, Br2+?

A, B, C, D
3. (Isotopes, natural abundance, probabilities, mass
spectrometry) Another diatomic molecule is H2:

The two nonradioactive isotopes of hydrogen are H and D, with ~99.9 %
and ~0.01 % natural abundance, respectively. Which combination is most likely
to be found in nature?
H2, HD, D2
At how many amu is the largest peak expected in the mass spectrum?
2, 3, 4
4. (Extended structures, discrete molecules; Table 3.1
"Companion")
Demonstration: HgO(s) ---> Hg(l) + 1/2 O2(g)
Is HgO an extended structure or discrete molecule?
Is O2 an extended structure or discrete molecule?
5. (Unit cells; Ch. 3 "Companion") Which of the
parallelograms in the figure below are unit cells?

A, B, C, D, E
After a unit cell is identified: What number of atoms belong to the unit
cell?
1, 2, other
6. (Unit cells; Ch. 3 "Companion;" empirical
formula) Pictured below is an NaCl layer. Which of the squares are unit
cells?

A, B, C
7. (Unit cells; Ch. 3 "Companion") A corner
atom is part of how many unit cells? At-Seat Demonstration: cubes
like dice can be handed out for students to examine for this question.

1, 2, 4, 8
8. (Coordination number; Ch. 5 "Companion")
In the two-dimensional square packing shown, what is the coordination number
of the central atom?

2, 4, 6
9. (Coordination number; Ch. 5 "Companion")
At-Seat Demonstration: Give students 10 pennies (students can share
a set of ten) and ask them to pack them at their desks to determine the
largest coordination number in two dimensions.
4, 6, 8, 9
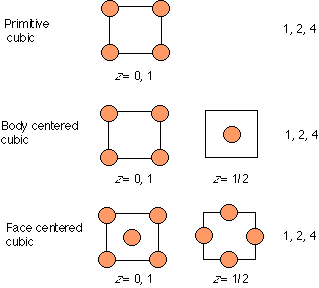
10. (Unit cells, layer sequences; Ch. 3 "Companion")
In the figures below, how many atoms per unit cell are present?
11. (Stoichiometry, empirical formula, elemental analysis,
atomic weights, magnetism) Demonstration 10.1 "Companion":
On an overhead, show that with a magnet, nickel powder is separable from
the aluminum powder. Show that the pressed bar comprising Ni and Al powder
is also magnetic using the overhead projector.
The reaction goes from face-centered cubic Ni and Al to produce an alloy
of Ni and Al with the following layer sequence:

What is the empirical formula of the product?
NiAl, NiAl2, Ni2Al, Ni8Al
Run the reaction and show that the product bar is less magnetic using
the overhead projector.
Will the elemental analysis be the same for the bar before and after the
reaction?
Same, Different

Al atoms weigh about one-half of Ni atoms:
For a complete reaction (Ni + Al ---> NiAl), roughly what mass ratio
of reagents should be used?
equal masses of Ni and Al
half the mass of Al as of Ni
twice the mass of Al as Ni
(An analogous set of questions could be developed for the quantitative reaction
of Fe and S.)
12. (Atoms, ions) How many atoms are in the formula Al2(SO4)3?
3, 5, 17
How many moles of ions are there per mole of Al2(SO4)3?
2, 3, 5
20. (Ionization energy) A(g) + energy---> e- + A?(g).
What is the charge, if any, on the product A?
+1, 0, -1
39. (Specific heat, heat capacity; Ch. 2 "Companion")
Setup: In solids, heat capacity near room temperature often reflects the
number of atoms in the solid. Demonstration 2.1 "Companion":
Heat equal masses of Al and Pb in boiling water. Transfer the Al to a beaker
of room temperature water. Measure the temperature increase with a digital
thermometer. Before repeating with Pb transferred to a separate beaker of
the same volume of room temperature water, ask whether the temperature change
will be greater with Pb, less with Pb, the same with Pb
42. (Elemental analysis, diffraction, solid solutions;
Ch. 3 & 4 "Companion") Sample A is an equimolar physical mixture
of Si and Ge. Sample B is a Si0.5Ge0.5 solid solution.
Which measurements will be identical and which different for the two samples?
elemental analysis: same, different
x-ray diffraction: same, different
absorption spectrum: same, different
43. (Vapor pressure) Demonstration: Drops of water and
ethanol are placed on an overhead projector and the ethanol drop is seen
to evaporate more rapidly. The graph below compares the vapor pressures
of ethanol and water. Which curve corresponds to ethanol?

A, B
Demonstration: Syringes are used to bring drops of H2O
and ethanol to the top of columns of Hg, as shown below. What will happen
to the heights of the Hg columns?

both column heights will be the same
the column below H2O is shorter
the column below EtOH is shorter
50. (Layer sequences; Ch. 3 & 5 "Companion")
How many atoms are in the following layer sequence of the diamond structure?

4, 6, 8, 13
What is the coordination number?
4, 6, 8
What is the coordination geometry?
tetrahedral, square, octahedral, cubic
53. (Layer sequences; Ch. 3 & 5 "Companion")
What is the empirical formula of the solid represented by the following
layer sequence?

LiO, LiO2, Li2O, Li8O14
65. (Unit cells, layer sequences, close packed spheres;
Ch. 3 & 5 "Companion") A layer sequence for an FCC = CCP metal
is shown below.

A face diagonal passes through the center of atom 4 and the center(s)
of which other atom(s)?
1, 2, 5, 11 Also correct: 8, 12 & 9, 10
A body diagonal passes through the center of atom 4 and the center(s) of
which other atom(s)?
2, 5, 11, 14
A close-packed plane is comprised of six atoms. If atoms 2, 4, 5 are
three of the six atoms, which other three atoms are need to define the plane?
11, 13, 14; 6, 9, 13; 7, 8, 12; 6, 9, 10
89. (Isotopes) The radioactive isotope 14C has how many
neutrons?
6, 8, other
107. (Isotopes, elements) The identity of an element is
determined by the number of which particle?
protons, neutrons, electrons
149. (Chemical formula) An elemental analysis shows a
sample of buckyball to be 100% carbon, and its mass spectrum shows a parent
peak at 720 amu. The formula would be written
60 C, C60, C




More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..
![]()







![]()




