a) Will the concentration of Cl- in solution increase or decrease?
b) Will the concentration of Pb+2 increase or decrease?
a) If some solid NaNO3 is added, will the solubility of CaSO4 increase, decrease, or stay the same?
b) If some solid Na2SO4 is added, will the solubility of CaSO4 increase, decrease, or stay the same?
c) If some solid CaSO4 is added, will the solubility of CaSO4 increase, decrease, or stay the same?
a) Is the charge on the surface of colloidal Ag(SCN) positive or negative?
b) What ion is the source of this charge?
Ag+, NO3-, K+, SCN-
c) Which ion predominates in the solution layer next to the particle?
Ag+, NO3-, K+, SCN-

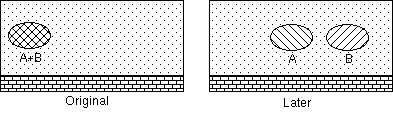
A, B, neither
1 extraction with 100 mL
2 extractions with 50 mL
5 extractions with 20 mL
![]()
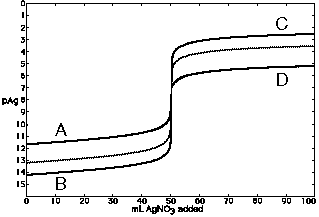
a) How will the titration curve change when the Ksp is larger (such as
a change from
10-9 to 10-7)?
A, B, or no change
C, D, or no change
b) How will the titration curve change when the solutions are equally more concentrated?
A, B, or no change
C, D, or no change
a) at the start? CH3COOH, CH3COO-, K+, OH-, H+
b) halfway to the equivalence point? CH3COOH, CH3COO-, K+, OH-, H+
c) at equivalence? CH3COOH, CH3COO-, K+, OH-, H+
d) after equivalence? CH3COOH, CH3COO-, K+, OH-, H+
4, 7, 10
HCl and NaOH
HCl and CH3COOH
HCl and CH3COOK
NaOH and CH3COOH
NaOH and CH3COOK
CH3COOH and CH3COOK
thymolphthalein (pKa 9.9)
bromothymol blue (pKa 7.10), or
bromocresol green (pKa 4.66)
![]()
![]()
![]()
More questions are needed for this section. Please submit contributions
to concept@chem.wisc.edu..