Informal Groups in Large Lecture. (Art Ellis, ellis@chem.wisc.edu)
There can be particular concern with the initial use of ConcepTests if it
is the first time "the class is turned over to the class." Many
of the ConcepTests on this web site have been used in large-lecture introductory
general chemistry classes of both majors and nonmajors of typically 200
to 350 students, and in recitation sections of about two dozen. Introducing
ConcepTests on the first day of class works well, although there is no reason
why the introduction of the method could not occur at any time during the
course. A typical introduction has been to pick an engaging topic, pose
a question, and provide two to four choices to stimulate discussion. Of
course, it is important to ensure beforehand that students are seated near
enough to one another to be able to converse.
As an example, an equation is written on the board and the instructor asks,
"Is this equation balanced?" After giving the class a few moments
for reflection, a show of hands is requested: "How many of you think
the equation is balanced? How many of you think it is not?" Usually
some hands are observed for each answer. There are also students who don't
commit to either answer. At this point the lecturer can say: "I'd like
you to turn to your neighbor, introduce yourself, and then convince him
or her that your answer is correct." There may be a moment of stunned
silence, as though the class is thinking, "You mean, we can talk in
class?" Then, typically, loud discussion ensues.
The intensity level of class discussion that follows often determines how
much time to allot for each question. After a suitable period of time has
passed, typically marked by a lull in the discussion level, the instructor
interrupts and asks for another vote. If by show of hands most students
have converged on the correct answer, the instructor can briefly affirm
why it is correct and move on. If the class has converged on the wrong answer
or not many hands are raised, this is a signal that the class is not following,
and the teacher has some choices. One choice is to provide an additional
clue, if the question lends itself to that, and repeat the process, with
or without a discussion period; or, the instructor can try to explain why
another answer is more appropriate. The value of the ConcepTest, as noted
above, is that the pace of the course is adjusted on-line as class mastery
is assessed in real time.
Formal Groups in Large Lecture. (Grant Krow, krow@chem.wisc.edu,
)
Grantkrow@aol.com
At the beginning of the term, a short examination based upon general chemistry
knowledge is given to each student. The results are used to assign students
to permanent groups whose members are heterogeneous to some extent by ability,
gender and race. A formal group size of five students has been found to
be advantageous in dealing with the organizational component of ConcepTests
and other group problem-solving techniques. In organic chemistry classes
of 100-200 persons in large lecture halls, we have found that five-person
groups of two students in one row and three in the neighboring row can conveniently
interact. The formal group structure allows students to know immediately
where to look for support and interaction when problems are posed for the
class. Discussion of ConcepTests is facilitated as students become relaxed
with their peer groups. Because students report out for their groups rather
than as individuals, pressures created by the desire to avoid "wrong"
answers is alleviated. We have found enthusiastic participation even when
we ask for student answers prior to giving a "desired" answer;
students understand that ConcepTests can be a time to problem solve. For
example, major concepts in a chapter on alkynes include the acidity of alkyne
hydrogens, conversion of terminal alkynes to ketones, and keto-enol equilibria.
A more advanced ConcepTest question, which required students to utilize
all three concepts, asked whether 1-propyne could be converted to 1-D-acetone.
Following group discussion, the groups who believed they could do the synthesis
reported their method (deuterium exchange followed by mercury-catalyzed
hydration of the alkyne) to the class on the board. This was followed by
voting. "Will this work? Should we review the mechanism?" Further
discussion of the mechanism by the students led to the insight that the
keto-enol equilibrium during the hydration step would wash the deuterium
out of the molecule so that the method would fail. Groups who had suggested
the initial synthesis were easily able to change their answers as they integrated
their knowledge of the keto-enol concept with their prior understanding
of reagents and pathways for alkyne reactions.
Report Out. (Clark Landis, landis@chem.wisc.edu)
Even in large lecture sections, ConcepTests provide a mechanism for easing
students into interaction with the class at large. By extending the vote/discuss/vote
sequence to vote/discuss/vote/report, students gain the opportunity to hear
the reasoning of other students expressed in their own terms. Imagine that
you have tried a ConcepTest to which the initial vote was split among two
or more answers. After discussion with their neighbors, a second vote leads
again to mixed vote. At this point the instructor asks for quick reports
of student reasoning for the correct answer only ("The correct answer
is A, would someone who chose this response explain why?") or may ask
for reports from two groups ("Both answers A and B received significant
votes, would someone who chose A please explain why? Now someone who chose
B?). In practice it is paramount that the classroom supports student reports
in subtle ways. Students are far more likely to report out if they have
discussed their answer with a group first; reporting out requires that the
instructor support group discussion in lecture (and live with the cacophony
and apparent loss of control). If the instructor chooses to have reports
only on the correct answer, first tell the class the correct answer. Given
the security of knowing that they are right, more students will volunteer
to report. At the conclusion of the report the instructor should paraphrase
the students' responses with an emphasis on the critical elements of their
reasoning.
Reporting of the reasoning behind both correct and incorrect choices is
riskier: students who explain an incorrect answer risk feeling publicly
humiliated. DO NOT tell the students the answer before asking for reports:
Who wants to explain publicly why they were wrong? Again paraphrase the
reasoning by both groups, emphasizing the common elements of the two responses.
Then reveal the correct answer and the reasoning. In some instances, the
wrong choice will reflect an incorrect assumption, not poor logic. Praise
the correct use of reasoning and point out the importance of checking assumptions.
BE SUPPORTIVE IN ALL THAT YOU DO. LEARNING IS A JOURNEY IN WHICH WRONG TURNS
ARE INEVITABLE. Extending the ConcepTest to public reports of reasoning
can lead the entire class to deeper understanding of the thought processes
that lead to correct answers and those based on common misconceptions. Use
this method wisely; important and difficult concepts benefit most from this
longer and more public application of ConcepTests.
Correct Answers to ConcepTests Are Rewarded with Bonus Points.
(Michal Moeller, mmoeller@unanov.una.edu)
Bonus points are given to all students if the class converges on the correct
answer to a Chemistry ConcepTest question. If the class splits evenly on
the answer then the entire class gets half of the original number of bonus
points.
Give a Student Group a Transparency to Project Their Answer.
(Art Ellis, ellis@chem.wisc.edu)
A small group of students is given a transparency on which they write their
answer to a ConcepTest question. For example, while the lecturer is demonstrating
a set of precipitation reactions at the front of the lecture hall, several
student groups are each assigned one of the reactions and are asked to write
its net ionic equation on the tranparency. The transparencies are then collected
and the class votes on whether each equation is correct or not. In this
method, the students providing the answers can remain relatively anonymous
minimizing embarassment if the group gives an incorrect answer.
Intensive Use in General Chemistry
(Judith Herzfeld, herzfeld@binah.cc.brandeis.edu)
ConcepTests were designed to step sequentially through the material without
any traditional lecturing. Students were expected to read an assigned part
of the textbook before each class and follow up with traditional assigned
problems from the textbook after each class. (The latter were discussed
during recitations, not during "lecture" hours.) During the class,
explanations were given only in the context of a specific ConcepTest, as
the instructor's side of a dialog, first introducing a question and later
summarizing the point. Students responded by using 4-lettered and -colored
signs (the colors serving to aid long distance recognition), rather than
a show of hands. The signs allowed a quicker pace, since all students could
respond at once. The signs also minimized self-consciousness about wrong
answers and allowed the instructor to coax those who hesitated to participate.
The hour typically started with easy questions, to establish basics from
the reading. Subsequent, more difficult questions then provided opportunities
for "peer instruction" (or, if necessary, instructor's leading
suggestions) for rethinking the problem. After the first thirteen hours
in this format, student opinion was surveyed. Out of a total enrollment
of 200, there were 160 respondents. The students agreed that ConcepTests
helped to keep them awake (83:17), were an extra incentive to come to class
(70:30), helped them to understand how to work with the material (81:19),
clarified concepts (76:24), are confidence building (62:38), helped them
to calibrate their understanding against the expectations of the professor
(83:17), and helped the professor to judge which concepts needed more explanation
and which less (85:15). Students were ambivalent about whether the ConcepTests
were "fun" and whether the teaching approach put too much reliance
on the reading. From the instructor's point of view, the frequent feedback
is extremely useful. Indeed, it is quite a revelation to realize how blindly
we fly when we lecture. It is also personally satisfying to offer the students
an experience that is less redundant with the textbook.
ConcepTests Used in Addition to Other Cooperative Learning Techniques
(Jeffrey Kovac, kovac@novell.chem.utk.edu)
Student active learning techniques, including ConcepTests, were used in
a large general chemistry course at the University of Tennessee, Knoxville,
during the fall semester of 1996. The course was the second semester of
a mainline general chemistry course based on the fourth edition of General
Chemistry by Darrell Ebbing. An absolute grading scale was announced at
the beginning of the semester. ConcepTests were used regularly during the
two weekly lectures. The number of ConcepTests varied depending on the material
covered, but at least one appeared almost every lecture. Sometimes, as many
as four were used during a 50-minute class. Some of the ConcepTests developed
for this course have been posted on the ConcepTest web site at the University
of Wisconsin-Madison. Other active learning methods used included cooperative
learning workshops during the teaching assistant-led discussions and cooperative
take-home exams.
At the end of the semester student reactions were surveyed using a five
point scale: strongly disagree through strongly agree. The relevant survey
questions and results were:
1. Having an absolute grading scale announced at the beginning
of the semester was helpful.
Strongly agree (16.1%) Agree (38.1%) Neutral (25.4%) Disagree
(15.3%) Strongly disagree (5.1%)
2. The "ConcepTests" used in the lecture helped me learn the
course material
Strongly agree (22.9%) Agree (44.1%) Neutral (23.7%) Disagree
(6.8%) Strongly disagree (2.5%)
3. The overall format of the course helped me learn chemistry
Strongly agree (11.9%) Agree (44.1%) Neutral (31.4%) Disagree
(9.3%) Strongly disagree (3.4%)
I found that the ConcepTests created a more interactive atmosphere in the
classroom. Most students responded positively, but there were a few that
defiantly sat alone and refused to participate in the discussions. On the
other hand, groups of students began to sit together because they found
the mutual interaction beneficial. They certainly were useful to me in providing
feedback on the crucial question: am I being understood? I am still getting
used to the technique, making decisions about what I can leave out of the
lectures that I have given for twenty years to allow time for the ConcepTests.
With a fairly standard departmental syllabus there are some difficult compromises
to be made. Overall, however, I have found ConcepTests to be a powerful
pedagogical technique and continue to use them in my teaching.
MultiStep ConcepTests (Chuck Casey casey@chem.wisc.edu)
In an organic chemistry course that consists of a lecture of 200-250 students, without discussion sections separate from the lecture, the use of a somewhat more complex problem with several steps as a ConcepTest offers students the opportunity to talk amongst themselves about not just one concept, but about several in succession, thus allowing them to put the pieces of the problem together. A synthesis question such as "Can this ketone be made selectively from an alkyne?" requires making connections between the concepts of oxidation, correct choice of starting material, and selectivity. The instructor can walk the students through the different steps of the problem by casting the problem as a series of more specific questions and soliciting discussion from the class at each point. Sometimes students offer suggestions for answers at each stage; usually the instructor offers suggestions, but not until after the students have talked amongst themselves and thought about the problem. This postponement of presenting the options removes the multiple-choice feel of the question and prevents the students from discussing only the possibilities that are alrady presented. These ConcepTests may take 10-15 minutes of lecture time.
The following pages of Chemistry ConcepTests contain what we refer to as type 1 and type 2 questions. Type 1 questions test whether a student has mastered an appropriate algorithm; type 2 questions require synthesis or extrapolation in order to answer the question correctly. Of course, the difficulty of the question depends on how much previous information the class has been given about a certain concept. Individual teachers must identify at what level the questions can be used.
Listed below are a few suggestions for writing ConcepTests. Some examples
illustrating the point are listed after each suggestion.
A. When possible include pictures, symbols, charts, etc. in addition
to words. (1, 15, 77)
1. (Isotopes) Consider the Br2 molecule. If there are two common isotopes,
79Br and 81Br, how many physically distinguishable combinations of Br atoms
are there in Br2?
![]()
2, 3, 4
15. (Limiting reagents, precipitation, solubility) Demonstration: A solution
of Ba(NO3)2 is added to a solution of Na2SO4 to make a precipitate. From
a table of solubility rules, the product is
barium sulfate, sodium nitrate
The amount of precipitate collected from the fixed amount of Na2SO4 solution
as the Ba(NO3)2 is added indefinitely will look like which graph below?
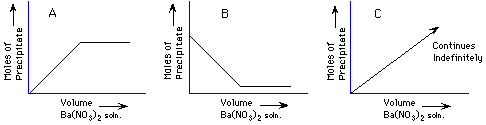
A, B, C
77. (Polarity) The following figure represents soap.
![]()
Which end of the anion is hydrophobic?
left end, right end
Will a soap solution be an electrolyte? Demonstration: Show that a light
bulb or LED with exposed leads lights up when the leads are immersed in
soapy water.
yes, no
Demonstration: Gently sprinkle lycopodium powder on top of a Petri dish
of water placed on an overhead projector. Take a toothpick, stick it into
a bar of soap, and then poke the toothpick into the center of the vessel.
The particles will be pushed to the edge by the soap molecules. Alternately,
a paper clip can be shown to float and then sink when soap is added.
How will the anions on the surface of a beaker of water prefer to arrange
themselves:
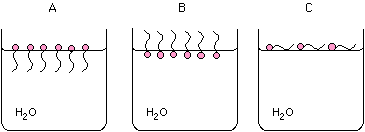
A, B, C
How does soap interact with grease (see below)?

A, B
B. Choose ConcepTests that focus on a critical concept and include common
misconceptions as possible answers. (12)
12. (Atoms, ions) How many atoms are in the formula Al2(SO4)3?
3, 5, 17
How many moles of ions are there per mole of Al2(SO4)3?
2, 3, 5
C. At a first pass, try to test a single new concept in each question.
(67, 69)
67. (Spectroscopy, probability; Ch. 8 "Companion") If a blue cupric
solution cuts the amount of red laser light reaching a solar cell, which
counts photons as photocurrent, in half...
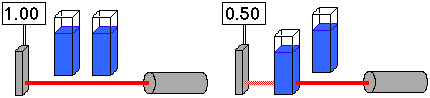
...what will happen if a second solution is added?
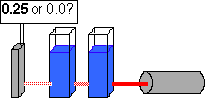
69. ( Weak acids) In acetic acid, pictured below, from where will the
H+ come?
![]()
O-H or C-H